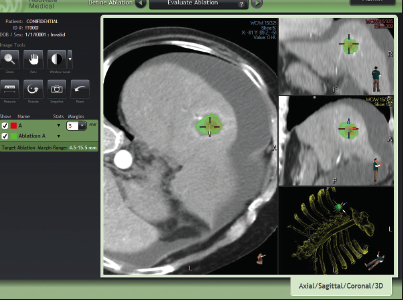
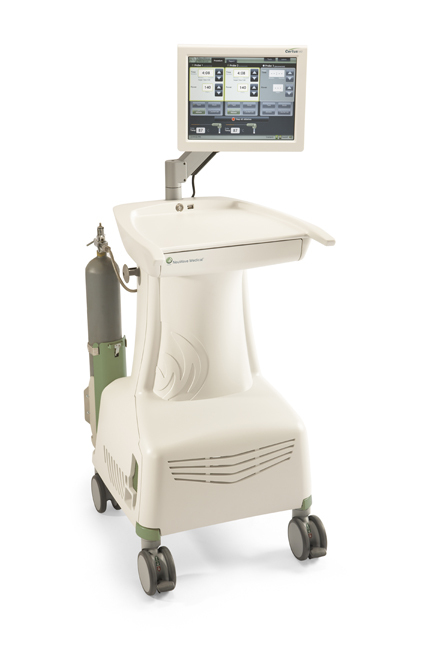
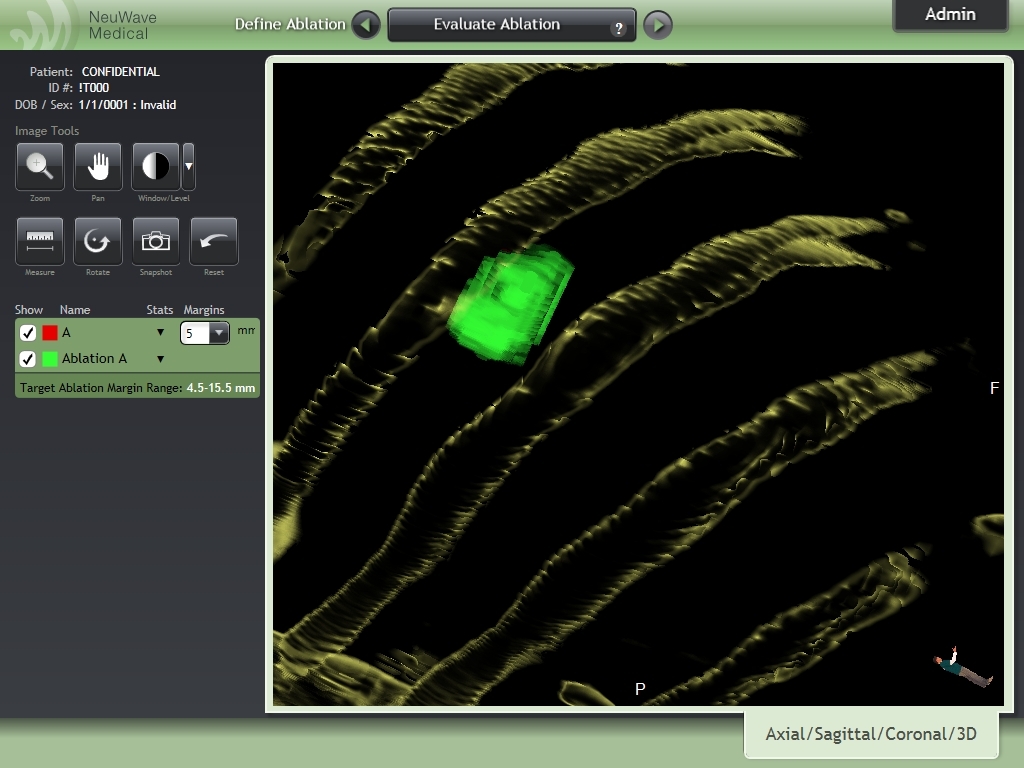
October 24, 2018 NeuWave Medical, Inc. Dan Kosednar Director of Regulatory Affairs and Quality Assurance 3529 Anderson Street Ma
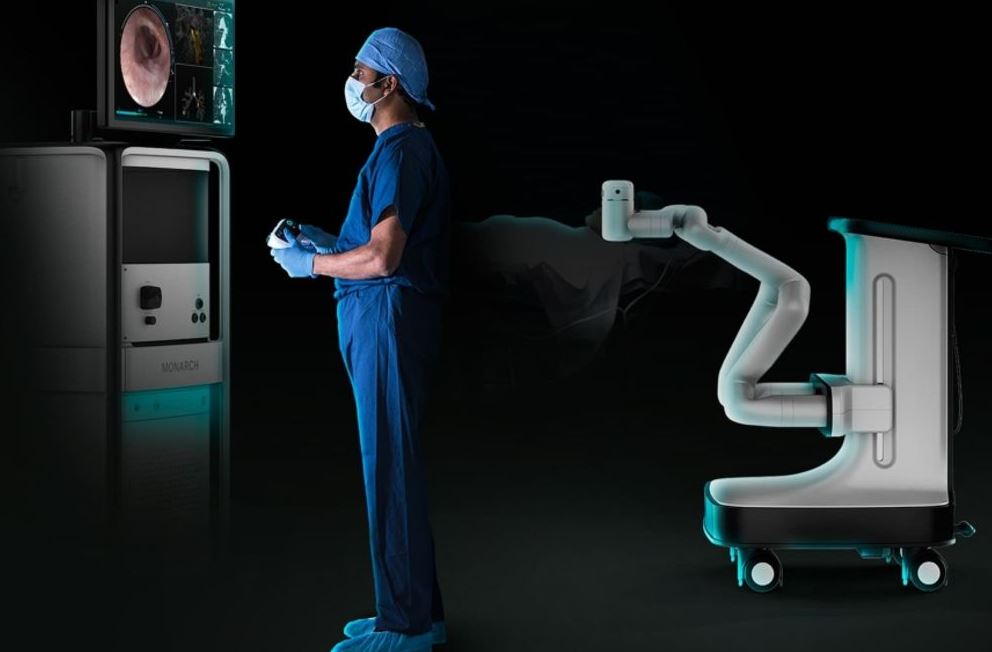
U.S. FDA Grants Ethicon Breakthrough Device Designation for Monarch-enabled NeuWave Microwave Ablation Technology | Legacy MedSearch - Medical Device Recruiters